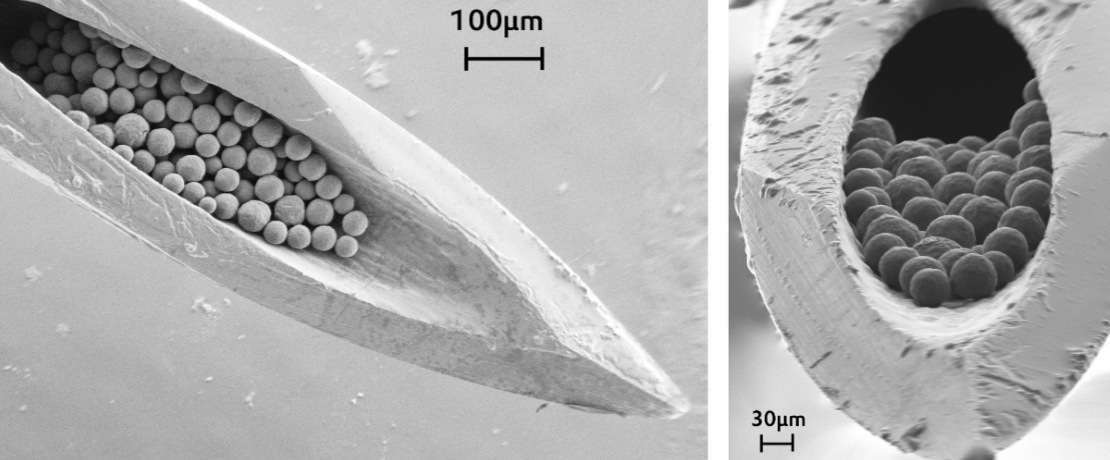
Is it time you knew more about biodegradable polymers for parenteral drug delivery?
Functional lactide/glycolide polymers play a key role in complex parenteral drug delivery products. They are often referred to as ‘smart’ materials, as their tunability drives the performance of parenteral dosage forms. Using these polymers, formulators can deliberately change drug impact by prolonging drug release in patients and offering long-acting or extended-release options.
Long-acting, complex drug delivery products, particularly those based on injectable microparticles, nanoparticles and implants, are among the most attractive parenteral products in the pharmaceutical industry – more than 50 products on the market worldwide. In fact, long-acting injectables (LAIs) that use bioabsorbable polymers for systemic and local drug delivery are receiving a lot of attention today, improving the lives of millions of patients globally, thanks to successful outcomes for the treatment of schizophrenia, diabetes, substance abuse, cancer, and ocular, rare and hormonal diseases. These products have many advantages, such as simplifying the drug regimen by decreasing dosing frequency, helping to improve efficacy, improving treatment adherence, and releasing beneficial agents locally, including in knee joints and within the eye.
Since the first synthetic plastic, Bakelite, was developed in 1907, the field of polymer science has grown drastically. Polystyrene, nylon, Teflon, and plexiglass were all invented in the late 1920s and 1930s. This is also the timeframe in which polylactide polymers were invented and, thus, are considered to be the origins of long-acting injectables – at least for those formulations based on lactide/glycolide polymers (LG polymers). However, the next significant development for LG polymers wasn’t until a 1955 patent. Up to then the advantages of these polymers that degrade in the presence of water had not yet been recognized; so they weren’t commercialized. The 1955 patent led to the first synthetic resorbable suture.
A lot has changed in the intervening years, with medical devices leading the way. Now LG polymers are an essential formulation tool and can be thought of as the timers or clocks of drug delivery, allowing tunable and precise uptake of water which influences drug release times so that they reach the most appropriate part of the body with maximum impact. Single or blends of LG polymers can be combined in dosage forms to match target release profiles over durations ranging from days or weeks to, in principle, more than a year.
So how do you use lactide/glycolide polymers to design these clocks and distribute the drugs for maximum effectiveness at the right time?
Polymeric chains
The key lies in their composition and molecular structure, such as understanding the glycolate blocks within the polymer chains. These blocks are part of the array of polymer properties that impact polymer solubility, formulation manufacturing and, ultimately, the drug release profile.
Polymer chains form when the cyclic monomeric lactide and glycolide species open up in the polymerization reaction – a ring-opening polymerization. A perfectly random polymer would include alternating incorporation of lactide monomer and glycolide monomer. Unfortunately, the monomers have little regard for the ideal polymer configuration. In real world conditions, when lactide and glycolide monomers are combined, mixed, and melted with catalyst and initiator, glycolide reacts ten times faster with itself as compared to the lactide monomer. The result is the formation of glycolate blocks along the polymer chain.
These glycolate blocks are not necessarily a concern in a single polymer chain. However, when several chains aggregate together, the glycolate blocks line up and form crystalline domains within the polymer matrix. As those crystalline domains form, they resist the integration of solvents and water into the polymer backbone. This impacts not only solubility of the polymer itself, but also the rate of water incorporation into the resulting formulation, which is how these polymers degrade and how they actually work in sustained release formulations.
Crystalline domains have a very low energy state, which means a high-power solvent is needed to dissolve “blocky” polymers. In fact, you could have two polymers, both with the same 50:50 lactide/glycolide ratio, but with completely different solubilities. Different solubilities lead to different processing conditions and different drug release profiles. It is, therefore, important to be careful about how these polymer chains are put together and, perhaps most importantly, to do so reproducibly so that large and complicated glycolate blocks do not form and impede solubility and change the way that the polymer degrades within the formulation. Polymer manufacturers’ proprietary polymerization know-how plays a key role in this regard.
Structure of monomers and LG polymers
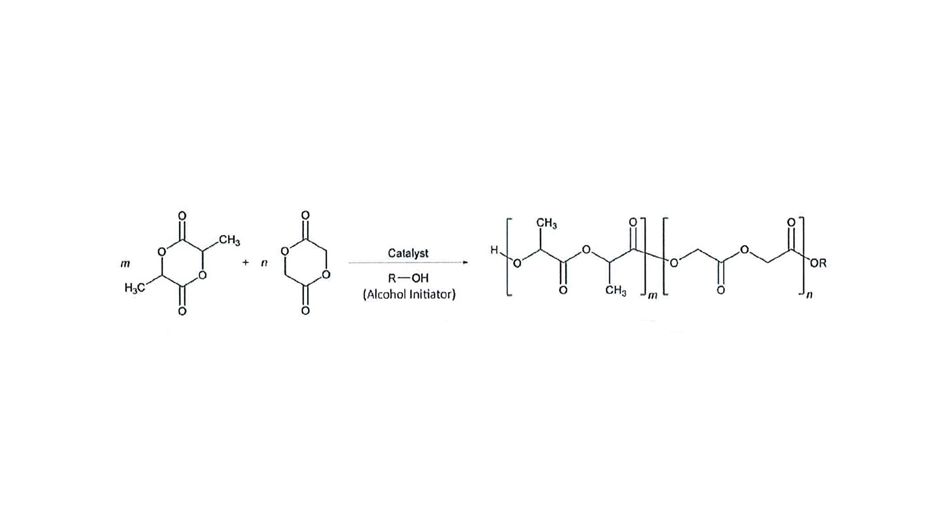
The lactide monomer (m) has methyl groups that are used to tune the hydrophobicity of LG polymers. Lactide methyl groups also contain asymmetric carbons. Most controlled release polymers use D,L-lactides, which include a racemic mixture of the two stereoisomers. Glycolide monomers (n) differ in that the lactide methyl groups are replaced with hydrogens. As discussed, increased incorporation of glycolide monomers can form blocks of crystalline regions within the polymer chain and can challenge polymer solubility.
What needs to be considered when you start formulating your long-acting injectable?
Most people first think about molecular weight as an important attribute. It is also important to consider the monomer ratio within the polymers—that is, how much lactide and glycolide are incorporated into the chain. While these are, indeed, critical attributes, one thing that is often neglected is block length and block length distribution during formulation development.
As formulation scientists began working with these polymers and trying to establish the origin of the observed solubility issues, an answer was discovered with carbon-13 NMR, which is capable of measuring the average glycolate block length within a polymer chain. This means we can now put a number on this attribute which is known to be quite important, and a number is something we can now control.
For polymers with upwards of 30% to 50% glycolide along the polymer chain, the glycolate block length attribute must be considered. As the observed average block length approaches three-and-a-half to four, solubility issues are likely to occur. While the number itself is important, one of most important considerations is that this number is kept consistent from lot to lot.
Follow the full discussion in the recording of our first episode of our fireside chat on "Polymeric drug delivery – long-acting injectables and beyond". It is available as on-demand video on our online platform onCare. A free registration for onCare is required.
About the authors
Tom Tice, Senior Director of Global Technical Marketing, has 43 years’ experience in bioresorbable polymers, microencapsulation, complex parenterals and drug delivery. Basically, 40 years of one polymer—lactide/glycolide polymers—and long-acting injectables. Over the past 17 years, he has also volunteered at USP, sitting on various expert committees. He’s also served for over 30 years in the McWhorter School of Pharmacy at Samford University, Birmingham, Alabama in the U.S.
Whitney Moro is currently the analytical and quality control manager for the LACTEL® polymer line within Evonik. With a background in small molecule synthetic organic chemistry, she has worked in the biodegradable polymer field for more than 10 years with experience synthesizing, scaling, analyzing, and formulating with poly(lactide-glycolide) polymers. She also volunteers with USP and resides on two expert committees.